ICCC (In-Country Clinical Caretaker)
Main duties
Before starting clinical trials
- Preparation of SOP for clinical trials
- Consulting and support for Japanese regulations
- CRO selection and assessing its reliability
- Preparation of Protocol, IB, ICF and other clinical trial documents
- Selection of PI and institutions
- Submission of Clinical Trial Notification (CTN)
- IRB submission
During the trial
- Monitoring study progress of the clinical trial
- Verification of compliance to GCP and protocol
- Gathering, provision and management of safety information
After completion of the trial
- CSR preparation and its reporting
Related Pages
In-Country Clinical Caretaker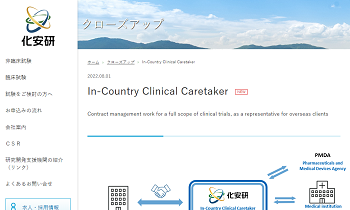 |
お問い合わせはこちら
~非臨床試験から臨床試験まで
ワンストップサポート~
試験のご依頼・ご相談はお気軽にお問い合わせください。